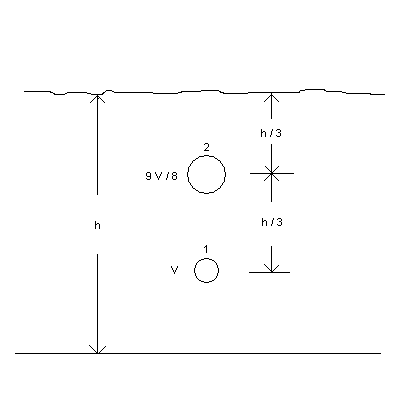
1. ( 20 points) A bubble submerged under water rises from position 1 to position 2 in the diagram below. The total depth of the water is h. As you can see, the bubble rises one-third of the total depth, to the position below the surface shown. At position 1, the volume is V and the temperature is exactly T 1 = 00C . At position 2, the volume is 9V/8 and the temperature is also exactly T 2 = 00C. Assume PATM = 1.013 x 105 N/m2. The density of water can be assumed to be exactly D = 1000 kg/m3 . Of course, assume that the bubble is so small that the pressure does not vary within its interior region. For maximum partial credit, use symbols to derive your answers, then plug in numbers at the last step.
| Solution: (a) P1V1/T1 = P2V2/T2 so that (Patm + 1000·g·2h/3)V= (Patm + 1000·g·h/3)·9V/8. Note that the temperature cancels. HOW NICE! Now solve for h to get h = 4.43 m. (b) P2 - Patm = 1000·g·h/3 (c) n = PV/RT, where you may use the values at 1 or 2. |

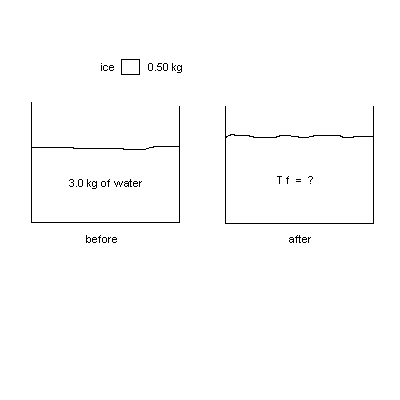
2. (20 points) At a party, a 0.50 kg chunk of ice at –10 0C is dropped into 3.0 kg of "tea" (water) at 200C. The specific heat of ice is Ci = 2100 J/(kg 0C), the heat of fusion of water is LF = 333000 J/kg, and the specific heat of water is Cw = 4186 J/(kg 0C). Assuming that the ice melts completely, what is the final temperature T f of the water ?
| Solution: MwCw(Tf - 20 ) = MiCi(0 - (-10)) + MiLF + MiCw(Tf - 0) so Tf = 5.1 0C. |
3. (26 points ) An ideal gas at an initial pressure of exactly Pi = 1.0 atm and an initial temperature Ti = 127 0C is taken through a process of expansion. The volume increases from an initial value Vi = 0.10 L to a final value of exactly Vf = 0.40 L. During this process, the pressure P varies inversely with the volume raised to the 3/2 power, according to the following formula:
P = 0.5·a·V -3/2
Note the – 3/2 for the negative exponent !
Also note: 1 L = 1x10-3 m3 , 1 atm = 1.013x105 N/m2 and R = 8.31 J/mol·K .
(a) ( 4 points) Find the final temperature. Give the answer as a number in degrees K.
(b) (18 points) Derive the general expression for the work done by the gas during this process. Show all steps. (This expression will be in terms of the symbols a, Vi and Vf )
(c) (4 points) What is the change in internal energy (in joules) during the process? Assume an ideal diatomic gas and that the molecules translate, rotate, but do not vibrate.
| Solution: (a) Find a = PiVi3/2/0.5, then find Pf = 0.5·a·Vf -3/2 then find Tf using PfVf/Tf = PiVi/Ti . Tf = 200K. (b) Integrate to get W = a·( Vi-1/2 - Vf-1/2) (c) delta U = (5/2)nR(Tf - Ti) |
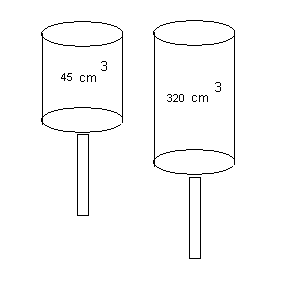
4. (extra credit) (7 points) During the power stroke in a four-stroke car engine, the piston is forced down as the mixture of gas and air undergoes an adiabatic expansion.
Check out the diagram below for this situation.
| Solution: See the hint and solution to problem 31, Quiz 3, Ch. 21. Same problem, just different numbers. |
Assume that:
1. the engine is running at 2500 rpm
2. the gauge pressure just before the expansion is 22.0 atm
3. The volumes of the mixture before and after the expansion are 45.0 cm3 and 320.0 cm3, respectively.
4. The time involved in the expansion is one-fourth that of the total cycle
5. The mixture behaves like an ideal gas, with gamma = 1.40
Find the average power generated during the expansion !
Hint:
![]()