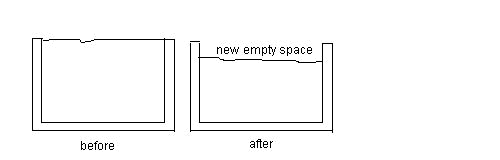
1. The average coefficient of volume expansion for moonshine alcohol
(illegal booze) is 5.750x10-4 (0C)-1.
Assume that a 60.000 gal steel container is filled to the brim with that
booze, and the container and alcohol are at 35.000 C (before) .
The linear coefficient of
expansion of the steel is 12.000x10-6 (0C)-1
. Suppose the moonshine and container are then put in a refrigerator and
cooled to 5.00 0C . (after)
What is the volume of
the new empty space between the top surface of the alcohol and the level
at the top of the container at 5.00 0 C (after) ?
![]()
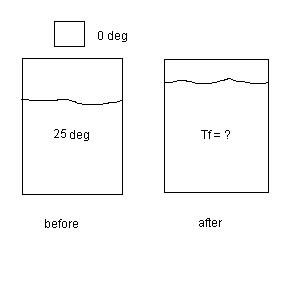
2.
(26 points) In an insulated vessel, 90.0 g of
ice at exactly 0 0C is added
to 800.0 g of water at 25.00 0C.
What is the final
temperature Tf of the water ?
See the diagram below for
this situation.
3. (20 points) During the compression stroke
in a four-stroke car engine, the piston
is forced up as the mixture of gas and air undergoes an adiabatic compression.
Check out the diagram below
for this situation.
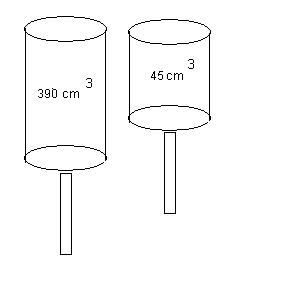
Assume that:
1.
the engine is running at
2600 rpm
2.
the gauge pressure just
before the compression is 2.00 atm
3.
The volumes of the mixture
before and after the compression is 390.0 cm3 and 45.0 cm3, respectively.
4.
The time involved in the
compression is one-fourth that of the total cycle
5.
The mixture behaves like an
ideal gas, with ![]() = 1.40
= 1.40
(a)
Find the average power
generated during the compression !
(b)
Extra Credit( 1 point)
What is the initial temperature Ti before the compression?
(c)
Extra Credit( 1 point)
What is the final temperature Tf after the
compression ?
(d)
Extra Credit ( 3 points)
What is the total change ![]() in internal energy of the gas mixture for the compression ?
in internal energy of the gas mixture for the compression ?