1.
(20 points) A person's lung of volume 0.50x10-3
m3 and internal temperature T = 17.0 0C contains air having an equivalent molar mass of
29.0 g /mol. The person then exhales
slightly and the volume decreases to 0.47 x10-3 m3 and the temperature increases
to 25.0 0C. Assume that the pressure inside the lung is constant at a gauge
pressure value of 1.50 atm.
What mass of air leaves that
lung ?
2.
(20 points) A thermal window with an area of 7.00 m2
is constructed of three layers of glass (G) 3.00 mm thick each separated from
each other by an air (A) space of 2.00 mm thick. See the diagram below. The
outside is mad cold at -30.0 degrees
Celsius, and the inside is nice and warm at 25.0 degrees Celsius . Kair = 0.024 W/m 0C and Kglass = 0.800 W/m 0C
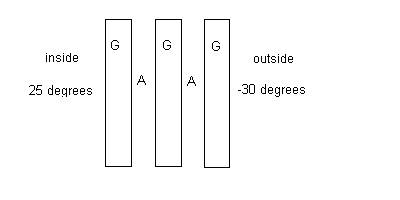
How long (in seconds) does
it take for 3000.0 Joules of heat to flow from the inside to
the outside ?
3. (26 points )An ideal gas
at an initial pressure of exactly Pi = 1.0 atm and an initial
temperature Ti = 273 K is taken through a process of
expansion. The volume increases from an initial value Vi =
3.0 L to a final value of exactly Vf = 20 L. During this
process, the pressure P varies inversely as the volume squared according to the following formula:
P = 0.5·a·V - 2
Note the -2 in the exponent
!
Also note: 1 L = 1x10-3 m3 , 1 atm = 1.013x105
N/m2 and R = 8.31 J/mol·K .
(a) ( 6 points) Find the final temperature.
(b) (11 points) Derive the general expression for the work done by the
gas during this process. Show all steps. (This expression will be in terms of
the symbols a, Vi and
Vf )
(c) (6 points) What is the change
in internal energy (in joules) during the process? Assume an ideal diatomic
gas and that the molecules rotate and vibrate.
(d) (3 points) What is the heat (in joules) transferred to the gas
during the process?
4. (4 points) EXTRA
CREDIT. 5 moles of an ideal gas has a
value of ![]() . In a constant volume process, what is the change in
temperature of the gas if 300.0 J of heat is added to the gas ?
. In a constant volume process, what is the change in
temperature of the gas if 300.0 J of heat is added to the gas ?